

【邀请函 | Inviting Letter】
当前未被满足的临床需求,药企研发策略应当如何选择?热门靶点扎堆、研究高水平重复该如何破局?应如何共同构建新药研发体系与创新生态圈?
亚洲医药研发领袖峰会(APRL)是亚洲地区聚焦药物研发主题的高端战略峰会。每年一届的研发盛会已陪伴中国与全球药物研发领袖走过十载光阴。第十一届APRL2022将再度汇聚来自全球跨国药企、国内外生物技术公司、科研院所等机构新药研发顶尖专家,深度聚焦以临床价值为导向、以患者为中心的药物研发与创新,共同致力于提升临床开发效率,加快新药开发上市速度,积极探索差异化研发战略,推动创新药研发国际化,连接全球药物研发领袖,携手攻克人类医学难题,向未满足的临床需求!
| In the face of unmet clinical needs, how should pharmaceutical companies choose their R&D strategies?How to break the current situation such as the gathering of popular targets and the research of high-level repetition? How to build a new drug R&D system and innovation ecosystem together? The 11th Asia Pharma R&D Leaders (APRL) is a high-end strategic summit in Asia focusing on drug discovery and development. This annual R&D event has been accompanying Chinese and global drug development leaders for ten years. The 11th APRL 2022 will once again bring together top experts in new drug R&D from global multinational pharmaceutical companies, domestic and international biotechnology companies, scientific research institutes and other institutions. APRL 2022 will focus on clinical value-oriented and patient-centered drug development and innovation,work together to improve the efficiency of clinical development, accelerate the speed of new drug development to market, actively explore differentiated R&D strategies, promote the internationalization of innovative drug development, and connect global drug R&D Leaders, work together to overcome human medical problems and unmet clinical needs! |
【论坛基本形式 | Basic Format For Summit】

【大会亮点 | Event Highlight】
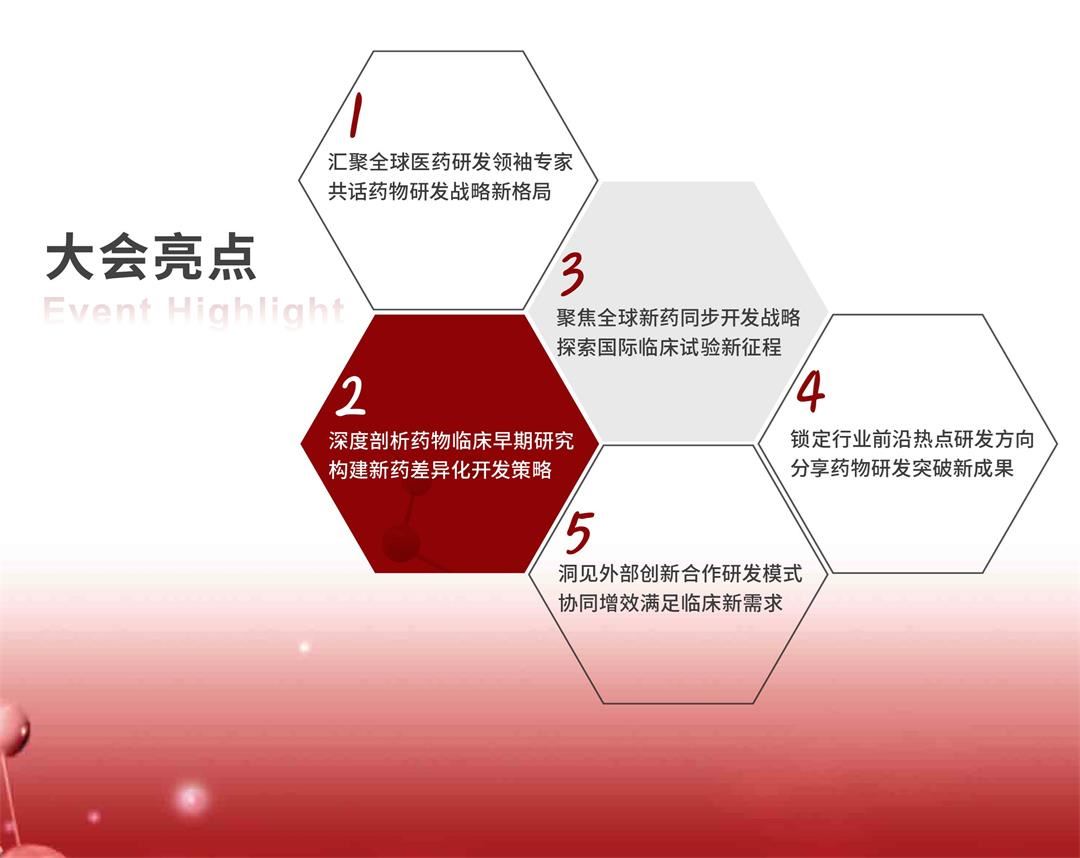
| |||
| |||
| |||
| |||
|
【谁将参与 | Who should attend】
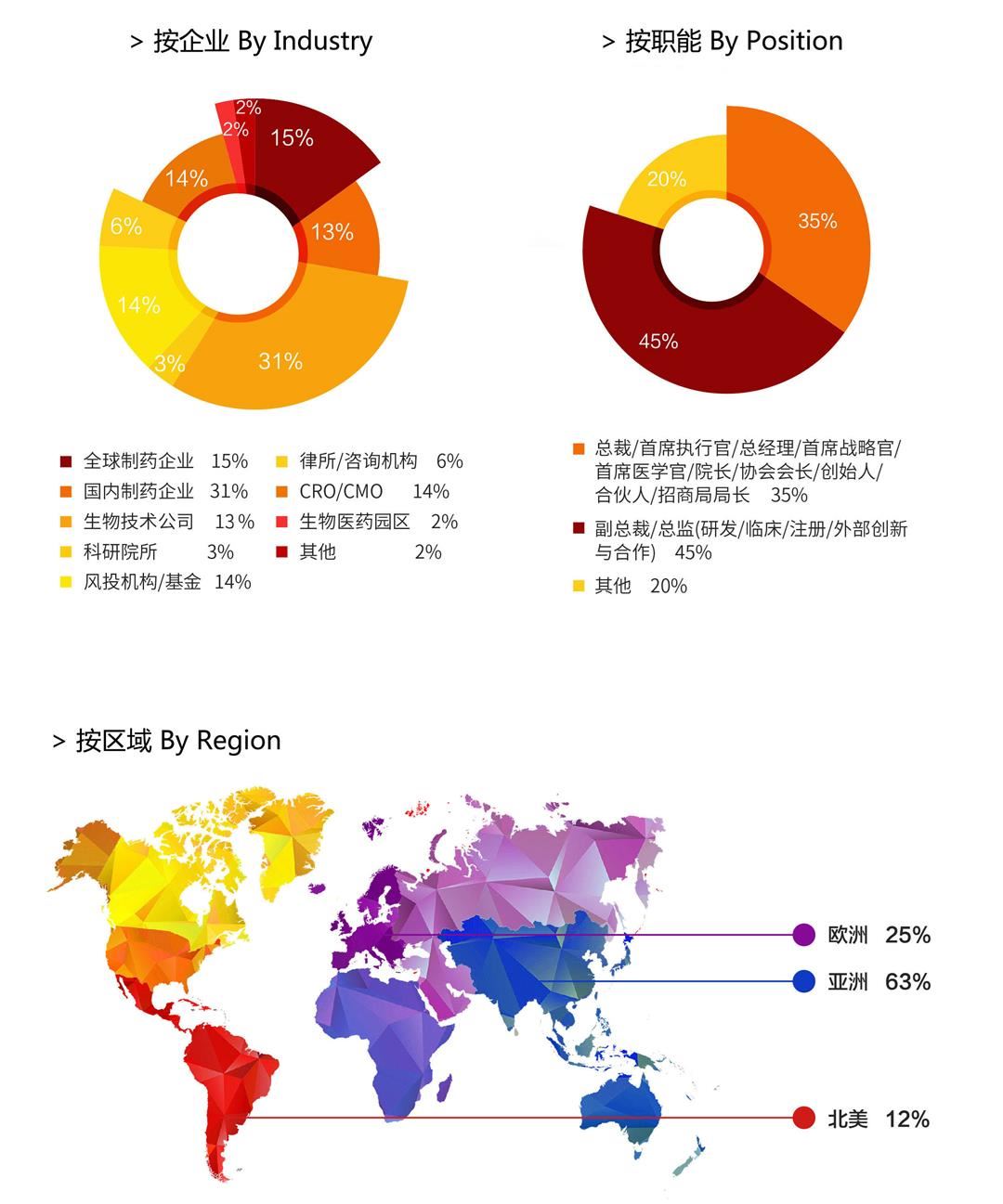
| Classified by Industries | ||
|
|
|
|
|
|
|
|
|
Classified by Titles | |||
| |||
| |||
|
【关键议题 | Key lssue】
| • 以临床价值为导向:中国药物研发与监管科学之路 • 新药开发全球化:海外药品审评审批政策与监管环境 • 全球研发格局:抗肿瘤药物的研发前沿进展 • 圆桌讨论:构建研发生态圈:探索加速产学研协同创新 • 新药早期研究开发策略与关键挑战 • 源头创新:靶点开发与新型化合物筛选 • 转化医学在新药早期研究中的应用 • 圆桌讨论:从中国新到全球新,中国医药创新生态系统的构建 • 全球临床合作:国际多中心临床策略的构建与实践 • 创新药临床试验设计关键要素与重点考量 • 全球新药同步开发下的法规注册与风险管理思考 • 圆桌讨论:经验交流,全球临床试验的困境与实践案例分享 • 双特异性抗体开发的关键与难点解析 • 下一个千亿市场:ADC抗体偶联药物的开发进展 • 新冠“特效药”竞速:中和抗体治疗性药物研发进 展与突破 • 圆桌讨论: 抗体研发格局,抗体药物差异化开发与布局 • 小分子药物研发策略及案例分析 • 小分子新药开发中的CMC关键策略与考量 • 创新药商业化高质量转化策略探讨 • 圆桌讨论:全球赛场竞技,小分子药物研发的差异化战略与布局 • 细胞与基因治疗产品工艺开发策略分享 • 国产mRNA新冠疫苗研发突破与产业化 • 新型AAV基因疗法开发与创新化探索 • 圆桌讨论:产业化之路,细胞疗法的商业化策略分析 • 中国医药产业投资并购的新格局与新趋势 • 创新药企投融资:多方合作的策略与考量 • 跨国药企在中国:药物研发模式与创新生态圈 • 圆桌讨论:协同创新,外部研发创新模式的构建与案例分享 |
| • Oriented by Clinical Value: The Science Road of China's Drug Development and Regulatory • Globalization of New Drug Development: The Policies and Regulatory Environment of Overseas Drug Review and Approval • The landscape of Global R&D: Frontier Progress in Anti-Tumor Drug R&D • [Panel] Building a R&D Ecosystem: Exploring and Accelerating the Collaborative Innovation of Industry, University and Research • Strategies and Key Challenges for Early Research and Development of New Drugs • Source Innovation: Target Development and New Compound Screening • Application of Translational Medicine in the Early Research of New Drugs • [Panel] From Chinese New to Global New: Construction of China's Pharmaceutical Innovation Ecosystem • Global Clinical Cooperation: The Construction and Practice of International Multi-Center Clinical Strategy • Key Elements and Considerations of Innovative Drug Clinical Trial Design Thoughts on Regulatory Registration and Risk Management under the Simultaneous Development of Global New Drugs • [Panel] Experience Exchange: Dilemma of Global Clinical Trials and Practice Case Sharing • Analysis of the Key and Difficult Points in the Development of Bispecific Antibody Drugs • The Next 100 Billion Market: The Development of ADC Drugs • Race of New Crown "Specific Drugs": Progress and Breakthroughs in Research and Development of Neutralizing Antibody Therapeutic Drugs • [Panel] The Pattern of Antibody Research and Development : Differentiated Development and Layout of Antibody Drugs • Strategy and Case Analysis of Small Molecule Drug Development • CMC Key Strategies and Considerations in the Development of New Small Molecule Drugs • Discussion on the High-Quality Transformation Strategy of Innovative Drug Commercialization • [Panel] Competition in the Global Arena: Differentiated Strategy and Layout of Small Molecule Drug Research and Development • Strategy Sharing of Cell and Gene Therapy Product Process Development • Development Progress of China's mRNA COVID-19 Vaccine • Development and Innovative Exploration of New AAV Gene Therapy • [Panel] Road to Industrialization: Analysis of Commercialization Strategy of Cell Therapy • The New Pattern and Trend of Investment and Mergers in China's Pharmaceutical Industry • Investment and Financing of Innovative Pharmaceutical Companies: Strategies and Considerations for Multi-Party Cooperation • Multinational Pharmaceutical Companies in China: Drug R&D Model and Innovation Ecosystem • [Panel] Collaborative Innovation: Construction of External R&D Innovation Model and Case Sharing |

【历届演讲嘉宾和VIP贵宾 | Previous Speakers and VIP 】



【历届参会企业代表 | Part of Previous Attending Companies 】


【历届会议精彩瞬间 | Wonderful Moment】

【联系我们 | Contact Us】
参会咨询和媒体合作请联系
For inquiries and media cooperation, please contact
Aili Li 李先生
TEL: (86 21) 6095 7201/13849919680(微信同号)
E-mail: aili.li@shine-consultant.com



-
2021年美国生药学学会年会(ASP)
2021年07月24日 阜新
- 2021年第60届毒理学协会年会暨博览会(STA 2021)
-
2021化药注册分类及申报资料要求剖析解读与CTD资料撰写
2021年07月08日 北京
-
T-Bio 2023第三届亚洲生物医药创新及质量科技峰会将于1月在上海召开
2023年01月05日 上海





